Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Function
- Solvent
- Industrial Additives Functions
- Technologies
- Product Families
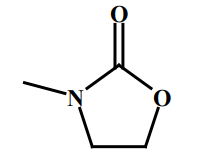
- Chemical Structure
Features & Benefits
- Industrial Additives Features
- Labeling Claims
- Product Overview
- E-GRADE® MEOX has excellent solubility, a relatively high flash point, low vapor pressure, and easy handling. It is an aprotic polar solvent that is versatile and easy to use.
- It is soluble in solvents and water. E-GRADE® MEOX is mainly used as a photoresist stripper and flash remover in semiconductor manufacturing
- Used for cleaning, post-etch residue removal (PERR) and Substitute for NMP in negative electrode slurries of lithium batteries and electrochromic
- It is also a suitable electrolyte solvent for EC.
- Benefits
- Ease of handling
- Excellent solvency
- Low vapor pressure
- Improved EHS profile
- Aprotic
- Water soluble and easily rinseable
- Stripping and cleaning
- Alternative to DMSO or NMP
- Product Benefits
- Easy to handle
- Excellent dissolving power
- Low vapor pressure
- Improve EHS (Environment, Health and Safety) aspects
- Aprotic
- Water-soluble and easy to rinse
- Peeling and cleaning power
- Alternatives to DMSO or NMP
Applications & Uses
- Applicable Processes
- Industrial Additives End Use
- Markets
- Applications
- Application Information
The alkanolamines and their aqueous solutions will absorb carbon dioxide and hydrogen sulfide at lower temperatures and release the acid gases at higher temperatures. This forms the basis for processes which separate carbon dioxide and hydrogen sulfide from gas streams.
Methyldiethanolamine is an alkanolamine used in tail gas treating and hydrogen sulfide enrichment units for selectively removing hydrogen sulfide from gas streams containing carbon dioxide. These units will, in most cases, permit 60 to 80% of the carbon dioxide to remain in the treated gas stream. Methyldiethanolamine is also used in natural gas plants for the bulk removal of carbon dioxide while producing a gas stream containing 0.25 grains hydrogen sulfide/100 scf. Bulk carbon dioxide removal can be realized with methyldiethanolamine when the CO2:H2S ratio ranges from 100 to 1,000.
Other suggested uses are urethane catalyst, textile softeners, pH control, and epoxy resin curing agents.
Properties
- Physical Form
- Soluble In
- Solubility Data
- The solubility of various materials was tested in multiple concentrations at 60°C, demonstrating that these solvents can be used as primary or cosolvents for these materials and offer alternatives to NMP. The maximum weight percent found to be soluble is listed below, however higher concentrations may be possible for some.
Regulatory & Compliance
Technical Details & Test Data
- Application Testing
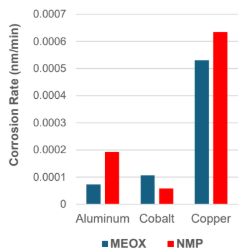
Low corrosion rates of both pure solvents confirmed by linear polarization resistance testing.
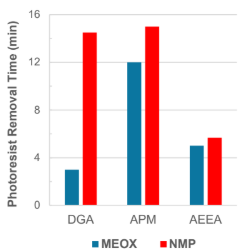
AZ1500 was post-treated and removed with a 50/50 blend of solvent and amine at room temperature. Results show MEOX had better removal times than NMP in all mixtures.
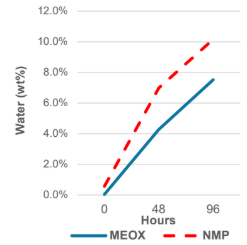
Multi-day study in high moisture environment indicates that MEOX absorbs less water from the environment than NMP.
- Solvent Photoresist Removal
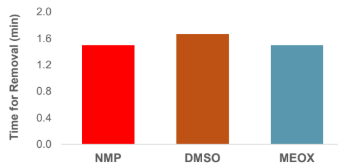
AZ1500 Photoresist removal at room temperature shows comparable performance of MEOX to other common solvents.

Positive Novolak photoresist patterned on 4 inch bare wafer followed by hard baking patterned wafer at 185°C for 2 min. The wafer was then stripped in solvent at 50°C for 5 min, and the photoresist pattern checked by microscope.
- Technical Information
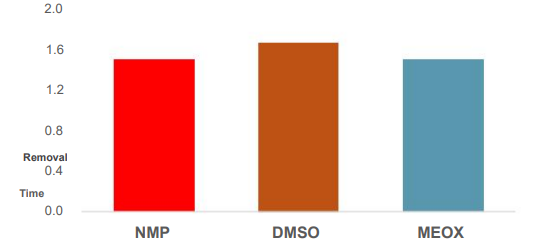
MEOX performed comparable to other common solvents in removing AZ1500 photoresist at room temperature.
- Test Data
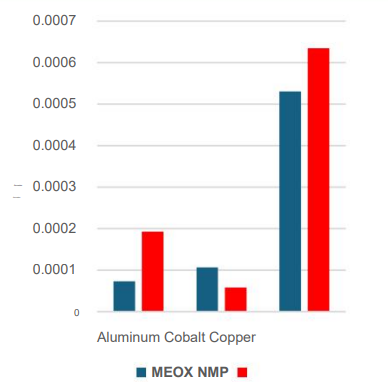
By the linear polarization resistance method, these pure. The corrosion rate of the solvent was confirmed to be low.It was possible.
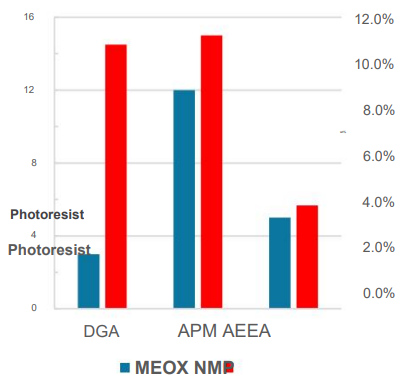
AZ1500 was stripped at room temperature with a 50/50 mixture of solvent and amine. MEOX was found to be Better removal time than NMP in mixtures I showed it.
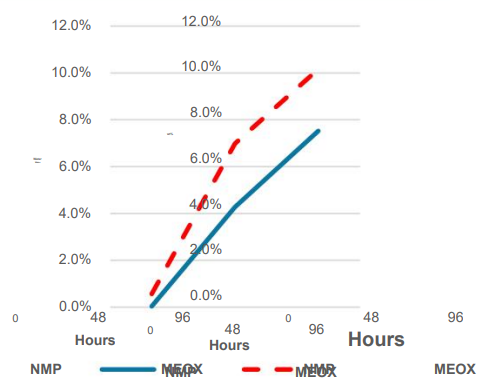
Storage test for several days in a high humidity environment As a result, MEOX was more effective than NMP in terms of external Less moisture is absorbed from the environment.
Safety & Health
- Toxicity & Safety
On the basis of acute studies with laboratory animals, methyldiethanolamine is considered slightly toxic by single oral dose and practically nontoxic by single dermal application. The oral LD50 value in the rat is 4.78 g/kg and the dermal LD50 value in the albino rabbit is 6.24 g/kg.
Methyldiethanolamine is considered moderately irritating to the eyes, but only slightly irritating to the skin. The product is not corrosive under the conditions of the DOT corrosivity test and is not regulated as a hazardous material for transportation purposes.
Because of the low vapor pressure of methyldiethanolamine, exposure to vapors is not expected to present a significant hazard under normal workplace conditions.
When handling methyldiethanolamine, chemical-type goggles must be worn. In addition, exposed employees should exercise reasonable personal cleanliness, including washing exposed skin areas several times daily with soap and water and laundering soiled work clothing at least weekly.
Should accidental contact with the eyes occur, flush them thoroughly with water for at least 15 minutes and get medical attention. Wash exposed skin areas with soap and water.
Packaging & Availability
- Regional Availability
- Availability Information
- Methyldiethanolamine is currently available in 55-gallon drums, tank wagons, and tank cars.
Storage & Handling
- Storage & Handling Information
The handling and storage of methyldiethanolamine presents no unusual problems. See the section on toxicity and safety for related additional information. The solvent properties and alkaline nature of methyldiethanolamine should be considered when installing handling and storage facilities. Methyldiethanolamine will react with copper to form complex salts, so the use of copper and alloys containing copper should be avoided. Carbon steel storage tanks, constructed according to a recognized code, are generally satisfactory.
Carbon steel transfer lines, at least 2 inches in diameter and joined by welds or flanges, are suitable. Screw joints are subject to failure unless back-welded because methyldiethanolamine will leach conventional pipe dopes. U.S. Rubber 899 gasket material or its equivalent is satisfactory for use with flange connections.
Centrifugal pumps are preferred with methyldiethanolamine, although carbon steel rotary pumps can be used. Rotary pumps should be equipped with externally lubricated bearings. A Durametallic Type RO-TT mechanical seal is suitable. Garlock 234, 239, or equivalent can be utilized as pump packing.
Other
- Appearance (SDS)
- Liquid
- Color (SDS)
- Light yellow
- Physical Properties
Value Units Test Method / Conditions Boiling Point 259.0 °C °C Flash Point 113.0 °C °C Closed Cup Melting Point 15.0 °C °C Molecular Weight 101.0 g/mol g/mol Viscosity 3.0 cPs cPs
